3 Lucid vs. non-lucid dreams
3.1 Non-lucid dreams
According to our analysis, non-lucid or “normal” dreams are characterized by low absolute values in all factors except REALISM. Non-lucid dreams seem almost to completely lack INSIGHT, CONTROL, and DISSOCIATION. Although mean scores for THOUGHT are higher than those for MEMORY, both are low if we are considering absolute values. Results also show relatively low mean values for NEGATIVE EMOTION. However, as most of our data were collected in a laboratory setting, known to increase positive emotionality in dream imagery (e.g., Hartmann et al. 2001), some caution is advised regarding the interpretation of results with respect to both negative and positive emotion.
3.2 Lucid dreams
Lucid dreams differ from non-lucid dreams in six of the eight factors identified in the LuCiD scale. The leading factor in lucid dreams is INSIGHT. Regarding the relevance of the other factors, we observed different rank orders for dream reports following sleep in a home setting (Figure 2a) and those from forced awakenings in the laboratory (Figure 2b). The data of our new laboratory study (Voss et al. 2014) confirm the findings depicted in Figure 2b, suggesting that the leading factors in dream lucidity are INSIGHT, CONTROL, and DISSOCIATION. Although, as pointed out by Windt (2013), dream reports in general must be considered trustworthy sources of evidence about subjective experience during sleep, the degree to which these reports can be used to draw scientifically sound conclusions about the dream state strongly depend on the quality of the experimental protocol. Such a protocol is more easily established in a laboratory setting, rendering immediate recalls of the dream experience, which must be considered more reliable with respect to distortions and intermixture with waking thought than those recorded in a home setting (Foulkes 1979; Voss et al., unpublished data), although dreamers might feel less inclined to report on sexual or aggressive content. Furthermore, reports from home settings usually lack information about the particular sleep stage (REM or NREM) in which the dream evolved. Typically, NREM dreams are less bizarre and more story-like (e.g., Dé Waterman & Kenemans 1993).
With regard to the distinction between primary and secondary consciousness in dreams, our findings indicate that INSIGHT is a defining feature of lucidity and that this core aspect of secondary consciousness is related to the emergence of other features of secondary consciousness. Lucid dreamers are able to reflect not only upon the fact that they are currently dreaming, but also upon the unfolding dream events.
The relationship between INSIGHT and CONTROL is clear, as realizing that one is dreaming is an important condition for trying to control not only one’s own behavior in the dream, but the dream itself. It must be pointed out, though, that CONTROL is much more infrequent than lucid INSIGHT, and the low covariance of this factor indicates a strongly limited variability of scores, suggestive of a floor effect. In other words, very few participants reported to have experienced some (however small) level of control over the dream plot (see Voss et al. 2013). Despite this limitation, lucidity appears to be characterized not only by lucid insight. INSIGHT also facilitates the emergence of other aspects of secondary consciousness in dreams such as dissociative thought and access to waking MEMORY. Similarly, while our study found non-lucid dreams to almost completely lack INSIGHT, CONTROL, and DISSOCIATION. THOUGHT, e.g., about other dream characters, was not completely absent in non-lucid dreams (Kahn & Hobson 2003).
A surprising finding of our study was that lucid and non-lucid dreams were not distinguished by a difference in the sense of REALISM. Whereas we previously thought that lucidity was characterized by a lack of bizarreness (see Voss et al. 2013), further exploration suggests that this factor is associated with the degree to which the dream feels real. Lucid dreams feel as subjectively realistic as non-lucid dreams. This finding was fully replicated in our most recent study (Voss et al. 2014). A question we are currently not able to answer is whether both dream types are equally bizarre (see also Windt 2013).
Our finding of realistic conviction stands in apparent contrast to reports from other authors who found that the onset of lucidity is often accompanied by a change in the overall experiential quality of the dream, noting that lucid dreams are often described as taking on a surreal, dream-like quality (cf. LaBerge 1985; Brooks & Vogelsong 2000; Tholey & Utecht 2000). At present, we are inclined to think that perhaps the different perceptions may be related to the already-mentioned confounding of wake- and sleep-induced lucid experiences. To our knowledge, lucid dreams entered through the wake state (e.g., Wake-Induced Lucid Dreaming, WILD, see Stumbrys et al. 2012) and those arising out of REM sleep have not been systematically compared with regard to phenomenology or Electroencephalography (EEG). Nonetheless, we think it plausible to assume that the WILD technique will result in more wake-like experiences, simply because they arise out of the wake state or the transition from waking to sleep, usually at the beginning of the night or after morning awakenings. A return to the wake state is in most cases easily accomplished. By contrast, dreamers who achieve lucidity out of REM sleep remain in REM sleep, not always being able to wake up voluntarily (Voss et al. 2009, 2014; Voss & Voss 2014). Regarding REALISM, lucid dreams arising out of REM sleep are apparently not accompanied by a change in the subjectively experienced realism of the dream.
3.3 Natural frequency of lucid dreams: The brain maturation hypothesis (1)
REM-sleep-induced lucid dreaming is unique because it represents an exceptional state in which the brain is in two states at the same time: awake and asleep. However, while many have experienced the phenomenon, few experience it on a regular basis. Why? So far, predisposing psychological variables have not been clearly identified (Schredl & Erlacher 2004). We have long speculated (Hobson 2009), and Schredl & Erlacher (2011) have confirmed, that lucid dreaming is negatively correlated with age. Why? And when does lucid dreaming actually set in? These questions need to be addressed in order to provide at least some clues about a very important question: Why does lucid dreaming occur at all?
To investigate the natural frequency of lucid dreaming in children and young adults, we interviewed almost 800 students aged 6–19. Students were recruited from local schools in and around Bonn, Germany, thanks to the enthusiastic cooperation of teachers and parents. Each student was interviewed alone, during school hours, and asked to provide a dream report and to answer questions about dreaming, lucid and non-lucid. In addition, to account for social desirability, students were tested for suggestibility (see Voss et al. 2013), which led to the exclusion of almost 100 data sets.
The main findings of our survey were a surprisingly high incidence of reported lucidity in the young and more frequent lucidity in those who are intellectually more capable. In total, 52% of participating students reported to have recalled at least one lucid episode in their life. The highest incidence rate of recent lucid dreams was observed in the young. Frequency rates seem to remain at steady levels until age 16, after which they drop dramatically.
In our study, only one third of lucid dreamers claimed to be able to change the dream plot, showing that plot control is not automatically activated in lucid dreaming. As in previous reports (e.g., Wolpin et al. 1992), plot control was significantly associated with frequency of lucid dreaming, suggesting that it is susceptible to training. Plot control was also found to vary with age. It remained at relatively high rates (up to 50% of lucid dreams) from 6 to 14 years and started to decrease from that age on. Lucid dreaming incidence or frequency was not related to sleep duration or napping.
Based on previous research into lucid dreaming, we are inclined to interpret these results as evidence that lucid dreaming is an exceptional mental state occurring naturally in the course of brain maturation. It is noteworthy that the peak in spontaneous occurrence of lucid dreaming coincides with the final stages of frontal lobe myelination and a time of synapse expansion and dendritic growth. These neurobiological changes provide the prerequisites for the integration of the frontal lobes (which are REM sleep-atypically activated in lucid dreaming) into the cortico-cortical and cortico-thalamic networks (Fuster 1989; Goldman-Rakic 1987; Zilles et al. 1988).
Lucid dreaming may thus occur naturally during the final stages of frontal lobe integration, a process similar to an upgrade of computer hardware. It seems to us most likely that the peak in spontaneous dream lucidity in childhood and puberty (Stumbrys et al. 2014; Voss et al. 2013) is nothing but an accidental confounding of conscious states during a time of high cerebral diversification. In an adult, mature brain system, relatively firm covariates for states of arousal have been established, For example, the frontal lobe activity during waking is usually enhanced, whereas it is down-regulated during REM sleep. Our Brain Maturation Hypothesis speculates that during childhood and puberty, frontal lobe activity is sometimes decoupled from the arousal state so that frontal lobes can become active in a state for which this type of activity is untypical. An intriguing finding is that not only lucid insight but also dissociative phenomena like derealization and depersonalization can easily be trained in the laboratory during this same period in ontogenetic development (Leonard et al. 1999). DISSOCIATION is a key factor that discriminates between lucid and non-lucid dreams (Figure 2, see also van Eeden 1969; Voss et al. 2013, 2014). In lucid dreams, dissociation is often described as taking on a visual third-person perspective, documenting a split between dreamer and dream observer (Gabel 1989; Rossi 1972) (“I see myself from the outside”), whereas non-lucid dreams are typically experienced from the first-person perspective, at least in adults (Foulkes et al. 1990; Gackenbach 2009; Snyder 1970; Voss et al. 2013). At this point, it may be important to note that we do not categorically differentiate between observer dreams and lucid dreams. Based on the results from our LuCiD scale study and in agreement with Gabel (1989), who speaks of “reflections of a dissociated self-monitoring system” (p. 560), we make a quantitative distinction between dreams experienced as first- or third-person, since DISSOCIATION is, next to INSIGHT and plot CONTROL, a key factor that discriminates lucid from non-lucid dreams (see Figure 2).
The fact that lucid dreaming is more readily experienced by those who are more advanced in abstract thinking and charged with reflective insight implies that lucid dreaming is indeed related to brain maturation. Support for this interpretation comes from a study by Lapina et al. (1998). Although details of method and sample characteristics have not been reported, the authors claim a higher level of lucidity in advanced learners. If this is true, however, then why does lucid dreaming decrease in early adulthood, considering that, surely, older students have acquired a higher level of abstraction than younger ones? At this point, we can only speculate about possible and probable causes. One explanation that should be further investigated is that lucid dreaming occurs naturally in the immature but developing brain.
Lucidity could thus be a transient dissociative state during brain maturation that is normally lost in adulthood but still accessible through training.
3.4 The hybrid state hypothesis (2) of lucid dreaming
The quantification of subjective experience in dream lucidity led us to assume that when the brain-mind shifts from non-lucid to lucid dreaming, it becomes a hybrid state with elements of both waking and dream consciousness. In lucid dreaming, thinking is only partially ruled by primary consciousness. To some extent, the dreamer has—however limited—access to secondary consciousness, enabling her to reflect on her present state. Aside from knowing that the on-going dream is not real, the dream is often experienced as if it were seen from the outside, almost as if the dream were an on-going theatrical production or motion picture (Voss et al. 2014).[1] In other words, lucid dreams can be considered dissociated states of consciousness in which the dream self separates from the on-going flow of mental imagery. The dream is still a dream, but the dreamer is able to distance him or herself from the on-going imagery and may even be successful in gaining (at least partial) control over the dream plot. This phenomenological dissociation is physiologically accompanied by highly selective increases in gamma band activity around 40 Hz in fronto-temporal areas of the brain (Dresler et al. 2012; Voss et al. 2009, 2014), while occipito-parietal regions retain a typical REM-sleep profile. For lucid dreams arising out of REM sleep, we have been able to document the maintenance of sleep throughout the lucid dream, suggesting that lucid dreaming alters REM sleep without surpassing it: REM sleep atonia is unchanged, rapid eye movement bursts (REMs) continue as in REM sleep. However, the EEG frequency spectrum is significantly altered (Voss et al. 2009). Normally, REM sleep dreams are accompanied by strongly attenuated activation and synchronicity in the gamma frequency band (Castro et al. 2013; Gandal et al. 2012; Voss et al. 2009), especially in frontal parts of the brain (Castro et al. 2013; Voss et al. 2009) suggestive of reduced conscious awareness and executive ego functions (Desmedt & Tomberg 1994). By contrast, gamma band activity in lucid dreaming is significantly increased, while all lower frequencies remain unchanged. This finding strongly suggests that sleep and even REM sleep is indeed maintained. Based on reports of our subjects on their lucid experiences we must assume, however, that lucid dreams push the arousal system towards waking while remaining within the region occupied by REM sleep and thus representing a substate located at the inner boundaries of the REM sleep area within the SoC. Lucid dreaming is, thus, a fragile, destabilized hybrid state. Several participants in our studies have stated that it takes effort to dream lucidly and that such dreams are easily interrupted by noise or state of mind.
Report of a lucid dreamer, f, 30 years old: “To me, being lucid is always a very exciting incident […] In this state it feels like a struggle in my brain between keeping the dream-scenery and waking. In these short periods of lucidity the awareness of the acting dream body and the real body in bed exist simultaneously and it costs a lot of concentration to keep the balance between both” (for further examples, see Voss & Voss 2014).
We also suggest that lucid dreaming is not just a hybrid state but actually the realization of two normally distinct global functions that usually don’t occur simultaneously. This fits in well with the common description of lucid dreams as (partial) awakening in our dreams and involving a split between dreamer and dream-observer, who coexist and change relative dominance of the mind at will (Occhionero et al. 2005). The implications of this line of reasoning have profound impact on the theory of mind. There are two selves, suggesting that the self is a construct elaborated by the brain (Metzinger 2003, 2009, 2013). The two selves of the lucid dreamer are mediated by distinct brain regions: dreaming is ponto-occipital while lucidity is fronto-cortical. Normally these two brain regions play a winner-takes-all game and dreaming is non-lucid. We come back to this point when we present our physiological model below.
We are attracted by the idea that a key cognitive component of waking, namely insight, can be admixed or even actively injected into REM sleep. Determining the degree to which this enhancement of lucidity is voluntary necessitates a better understanding of altered states of waking conscious awareness, such as hypnosis or mind wandering. We need to know more about conscious state control and to bring that understanding into conjunction with our attempt to understand and influence consciousness.
3.5 Space of Consciousness Model (3)
To speak of lucid dreaming as a hybrid state implies, of course, that states in general have boundaries and intermediates (so-called hybrids). We have, in a recent publication (Voss & Voss 2014) taken this thought further and proposed a model based on the assumption that consciousness is a dynamical process unfolding in a phenomenal state-space continuum occupied by states of arousal such as waking, sleep, and coma. Normally, waking and dreaming constitute two distinct partitions in this state-space. In our new model, what we have called the hybrid of lucid dreaming is depicted as a region within the state of REM sleep that stretches REM state variability to the point of destabilizing it, bordering on waking without inducing a complete change of the global configuration.
In our SoC model, we define consciousness as a three-dimensional space occupied by states that vary as a function of sensing, judging, and motor control. “Sensing” refers to the ability to experience physical and mental fluctuations. “Judging” is meant to describe varying degrees of higher-order cognitive capacities such as reflective awareness, including the ability to dissociate, to think about the past and contemplate the future, and make decisions. The “motor control” dimension was introduced to allow enough space to position different types of unresponsive states such as coma (low motor control, low sensing, and low judging) and, for example, locked-in-syndrome, which would be low in motor control but high in sensing and high in judging. Our model is even broad enough to include artificial intelligence (e.g., high judging and low sensing) and to span all forms of animal life as well (see Tononi 2004). Importantly, we do not differentiate between internal and external sources of information or state-dependent neurochemical modulations, as laid out in the AIM model (Hobson et al. 2000; for an early version see Hobson & McCarley 1977). Our space-state model is exclusively phenomenological. The main questions it addresses center around state boundaries and within-state variability.
The space is divided into subspaces, corresponding to states of arousal, such as waking, sleep, or coma. These States largely determine the ability to interact with the external world. We may think of this total space as originating at the near-death state, spanning over several stages of sleep and wakefulness to some ultimate wake-state of focused attention (see Figure 3). However, it should be kept in mind that the near-death state may not at all be one of minimal expressions of judging and/or sensing (Borjigin et al. 2013; Nelson 2014) so that another altered state may more accurately define the true origin of the SoC.
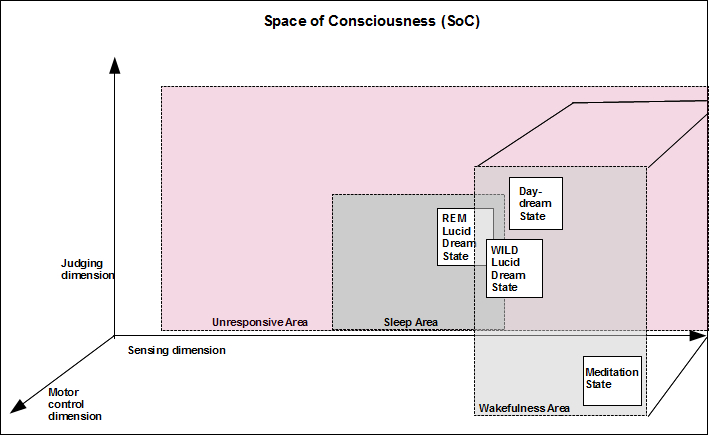 Figure 3: 3-dimensional Space of Consciousness Model (adapted from Voss & Voss 2014, p. 32).
Figure 3: 3-dimensional Space of Consciousness Model (adapted from Voss & Voss 2014, p. 32).
Lucid dreaming briefly creates a trajectory that dynamically integrates the region normally occupied by waking experiences with that of dreaming.
Each state, occupying some area within the SoC, can also be described by a finite number of attributes, and each state possesses a limited degree of variability. Within the partition characterizing wakefulness, for example, we find mind wandering, meditation, and hypnosis, as well as focused attention. Regarding lucid dreams, we assume that wake-induced lucid dreams can be represented by trajectories leading the system very close to the borders, but which still remain within the overall region inhabited by wake states, whereas REM-sleep-induced lucid dreams initially belong to the sleep state and then evolve towards a brief and unstable integration of the phenomenological substates of waking and dreaming.
Some new questions that derive directly from the model concern (1) the exact number of separable states; (2) specification of the sufficient and causally enabling (perhaps even necessary) conditions allowing for transition from one state into another; and (3) the volume and the dimensionality (the “depth”) of a given region in state-space characterizing each individual state, some perhaps extending over such a broad spectrum of conscious experiences that substates can be defined within the total of SoC and some occupying only a diminutive space such as coma. An example of a high-volume region in phenomenal state-space is wakefulness, covering a wide range of substates including WILD, mind-wandering, focused attention, and hyper-arousal. Another region is sleep, providing a smaller and more dimensionally limited, but nonetheless also considerable range of substates such as light sleep, slow wave sleep, REM sleep (both phasic and tonic), and lucid dreaming.
The SoC model is only an approximation, but we hope that it will prove useful in the generation and testing of specific hypotheses. With regard to lucid dreaming, we hope that this model will contribute to understanding and categorizing the many different aspects and conditions of insightful dreams such as those arising out of the wake state (WILD) versus those arising out of REM sleep. We would expect wake-induced lucid dreams to be accompanied by a much greater motor control, for example, than lucid dreams arising out of REM sleep, simply because WILD are located near the borders of the wake state whereas REM lucid dreams occur in sleep.
3.6 EEG changes associated with lucid dreaming
Our first quantitative EEG study on lucid dreaming aimed to identify changes in brain activity, provided they turned out to be measurable. For this purpose, we trained a relatively large group of students (N = 20) at Bonn University in lucid dreaming. Following several months of preparation, we took those who had achieved lucidity at home 2–3 times per week into the sleep laboratory at the Neurological Clinic of Frankfurt University Hospital.
Although our subjects were highly motivated, our hopes of being able to trace a multitude of lucid dreams soon had to be abandoned, since our enduring attempts yielded EEG recordings of only three spontaneous lucid dreams! Results of this meagre yield were published (Voss et al. 2009), showing that lucid dreaming occurs when activity in the lower gamma band around 40 Hz increases, particularly in frontal parts of the brain. In other words, the results suggested that normal dreaming is cognitively impaired because of frontal lobe deactivation and lucidity only occurs when that deactivation is suspended, either spontaneously or by design.
This finding is depicted in Figure 4, showing single subject 40 Hz EEG power (36–44 Hz) during waking with eyes closed (top), lucid dreaming (middle), and normal non-lucid REM sleep (bottom).
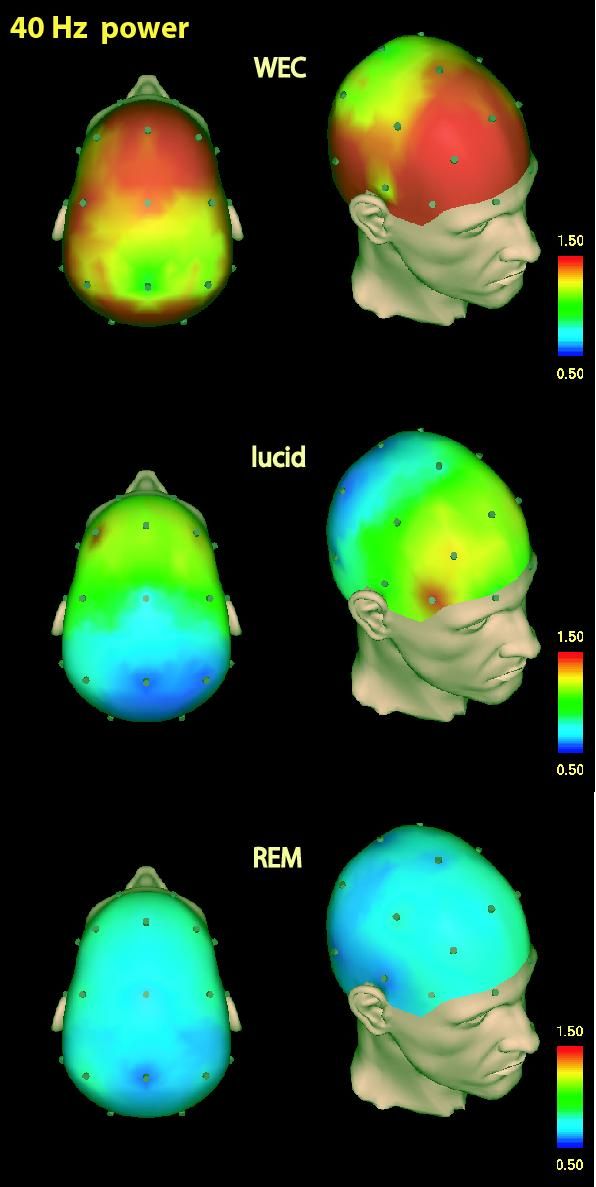 Figure 4: (adapted from Voss et al. 2009). Single subject 40-Hz standardized CSD power during Waking with Eyes Closed (WEC) (top), lucid dreaming (middle), and REM sleep (bottom). Topographic images are based on movement-free EEG episodes and are corrected for ocular artifacts.
Figure 4: (adapted from Voss et al. 2009). Single subject 40-Hz standardized CSD power during Waking with Eyes Closed (WEC) (top), lucid dreaming (middle), and REM sleep (bottom). Topographic images are based on movement-free EEG episodes and are corrected for ocular artifacts.
Another finding concerns EEG coherence, or synchronicity (see Figure 5). Whereas the coherence between different brain areas is high in waking (top), it is very low in non-lucid REM sleep (bottom). In lucid dreaming, however, it is significantly increased in comparison to non-lucid dreaming, especially between anterior and posterior parts of the brain (middle).
In this first study, we encountered several methodological difficulties.
-
For the subjects, achieving lucidity in a laboratory setting was difficult. In all three instances, lucid dreaming occurred in the late morning hours, i.e., after 8am. Our study was conducted in the sleep laboratory of the Neurological Clinic at the Frankfurt University Hospital. This implied a noisy early morning routine in which patients were frequently moved for examination purposes, breakfast was served, and floors were cleaned with heavy machinery. It is our opinion now that lucid dreaming arising out of REM sleep is a fragile state that can be easily disrupted by ambient noise.
-
Several authors have cautioned that some of the variance in gamma activity might be caused by microsaccadic eye movements (Trujillo et al. 2005; Yuval-Greenberg et al. 2008; Weinstein et al. 1991) and by scalp EMG activity (Whitham et al. 2008; Whitham et al. 2007). Although it is not known, at this point, whether microsaccades are present in steady-states, especially sleep, we have, for publication purposes, conducted a very conservative signal analysis using current source densities (Current Source Densities, CSD). By using this method, we may have overcorrected our EEG scalp potentials, which means that the actual increase in lower gamma band activity is probably even greater than reported.
-
Our subjects reported themselves to be less lucid in the laboratory than at home. When asked to specify this subjective rating, we found that the subjects’ responses were vague and mostly concerned with the amount of plot control achieved in the dream.
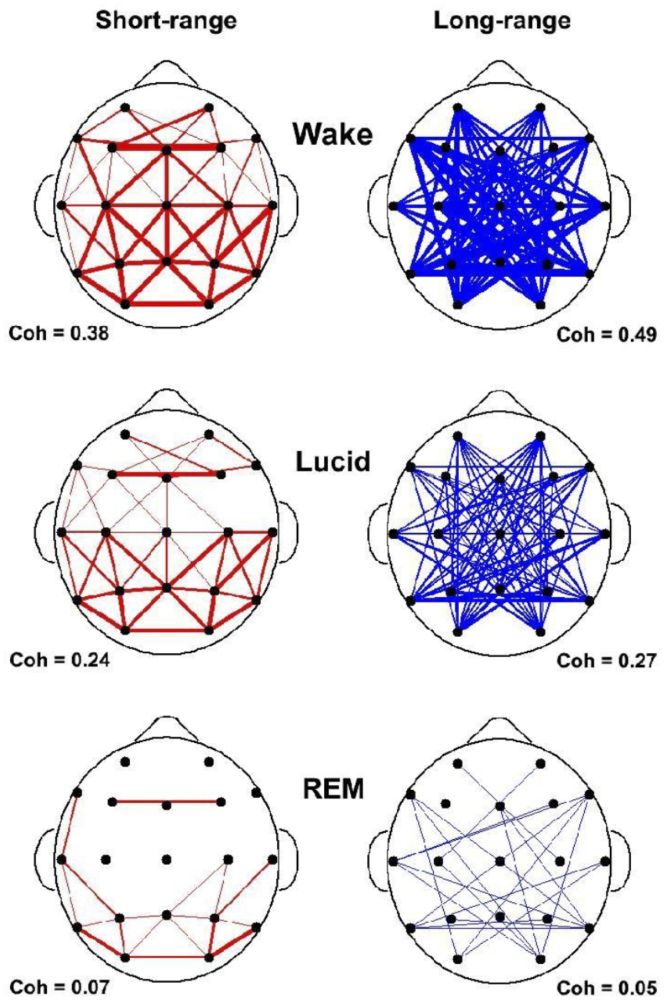 Figure 5: State-dependent short and long range coherences in the 40 Hz frequency band during Waking with Eyes Closed (WEC) (top), lucid dreaming (middle), and non-lucid REM sleep dreaming (bottom). Coherences are indications of interscalp networking and synchronization. Short-range (N = 55 pairs) was defined as less than 10cm and long-range (65 pairs) as larger than 15cm inter-electrode distance. Coherences are lowest in REM sleep and strongly enhanced in lucid dreaming.
Figure 5: State-dependent short and long range coherences in the 40 Hz frequency band during Waking with Eyes Closed (WEC) (top), lucid dreaming (middle), and non-lucid REM sleep dreaming (bottom). Coherences are indications of interscalp networking and synchronization. Short-range (N = 55 pairs) was defined as less than 10cm and long-range (65 pairs) as larger than 15cm inter-electrode distance. Coherences are lowest in REM sleep and strongly enhanced in lucid dreaming.
The findings of our 2009 study indicate that when subjects became lucid, they shift their EEG power, especially in the 40Hz range and especially in frontal regions of the brain. This shift is, in part, a consequence of pre-sleep auto-suggestion, indicating that REM dream consciousness, which is largely automatic (i.e., spontaneous, involuntary, and intrinsic), is partially subject to volitional force. This observation and its interpretation have an obvious relationship to the question of free will, an implication we will discuss later. Our speculative hypothesis is that dream lucidity arises when wake-like frontal lobe activation is associated with REM-like activity in posterior structures.
3.7 The gamma band hypothesis (4)
In our study of EEG tracings during lucid dreaming, the most striking finding was that lucidity was accompanied by an increased activation of the frontal lobes of the brain. This applies both to synchronicity and to consciousness-related frequencies (around 40 Hz). This observation has led us to propose a “gamma band hypothesis” (Voss et al. 2012; Hobson & Voss 2011), suggesting that brain activation in the 40 Hz frequency range is related to secondary consciousness. We have, in a recent study (Voss et al. 2014), investigated this hypothesis by fronto-temporal application of weak electrical currents in various frequencies. The study was aimed at testing for causality. If activity centered around 40 Hz was causally related to secondary consciousness as expressed in lucid dreaming, then the application of 40 Hz should induce lucid dreaming, provided that it is possible to change brain function in a frequency-specific way through mild electrical stimulation.
3.8 Induction of lucidity via electrical stimulation
In our latest study, we set out to test the hypothesis that lower gamma activity in the frontal and temporal parts of the brain causally enables lucidity during dreaming. If the observed gamma activity during naturally-occurring lucid dreaming plays a causal role in lucidity, we predicted that facilitation of that frequency band with 40 Hz transcranial alternating current stimulation (tACS) over fronto-temporal areas would increase the probability of lucid dreaming. On the other hand, tACS with a lower or higher frequency should have no effect or even suppress lucid dreaming. The current strength was kept below arousal threshold (250 µA) in order not to awaken the subjects. Participants were inexperienced lucid dreamers without psychopathology or sleep problems. They were not asked to try to have a lucid dream. Instead, they were told that the study goal was to investigate the effects of mild electrical stimulation in different frequencies on dream content and sleep parameters. While we were doubtful whether it was at all possible to enforce a specific rhythm on the brain (“driving fields”), results suggest that it is indeed possible to change brain activation in a frequency-specific way (see Figure 6). However, we only observed such an effect for frequencies within the lower gamma frequency band. Stimulation with higher or lower frequencies did not result in a measurable change in the respective frequency band, i.e., stimulation with 2 Hz did not lead to an increase in delta frequency band power.
Regarding lower gamma band stimulation, the induced change in lower gamma band brain activity was obviously potent enough to alter conscious awareness in the dream with increased LuCiD ratings especially for INSIGHT and DISSOCIATION. Again, this was not observed following stimulation with either higher or lower frequencies.
In this experiment, we tested twenty-seven healthy subjects, during up to four non-consecutive nights. Testing was conducted in a neurophysiologic sleep laboratory at Goettingen University Hospital. We tested during the summer break of the laboratory and on weekends, which provided a quiet environment and which allowed subjects to continue sleep beyond normal hospital wake-up hours. Participants were allowed to sleep uninterrupted during the first half of the night until at least 3am.
Starting at 3am, stimulation (30s long) was conducted during REM phases, and subjects were awakened shortly after this stimulation. At this time, they were asked to provide a dream report and ratings to all items of the LuCiD scale. The study was performed double blind, so that neither the subject nor the interviewer knew the stimulation frequency applied. In a repeated measures design, all participants were exposed to all stimulation conditions, i.e., sham (no current applied), 2 Hz, 6 Hz, 12 Hz, 25 Hz, 40 Hz, 70 Hz, and 100 Hz (details of methods, see Voss et al. 2014).
Note that we only applied tACS during REM phases, as lucid dreams arising out of REM sleep were our main research interest. Repetitive stimulation during other sleep stages would have exhausted the experimental protocol and would have led to many undesired side effects such as sleep-deprivation from repetitive early awakenings, changes in sleep architecture, carry-over effects from stimulation in other sleep stages, time-of-night effects, etc.
As shown in Figure 6, only stimulation in the lower gamma band, i.e., stimulation with 25 and 40 Hz, led to an increase in activity in this particular frequency band.
At present, we can only speculate why the other frequencies were not as easily adopted by the brain. Lower frequencies might not have been readily entrained because of state-dependency, as proposed by several authors (Buzsáki & Draguhn 2004; Vyazovskiy et al. 2009; Tononi et al. 2010; Brown et al. 2012; Suh et al. 2010). It is possible that if we had tried to induce a frequency typically enhanced in slow wave sleep (SWS), for example, such stimulation might have disturbed physiological sleep-dependent oscillations, which would prevent the brain from accepting such a driving field. This notion is supported by direct current (DC) studies (equivalent of 0 Hz) of brain stimulation in REM sleep (Jakobson et al. 2012a; Stumbrys et al. 2013). Both group of researchers were unable to alter on-going mental activity at 0 Hz, just as we were unable to induce lucidity at 2, 6, or 12 Hz. Interestingly, dream reports were less frequent in these stimulation conditions (Voss et al. 2014). However, this does not explain why stimulation with higher frequencies, i.e., 70 and 100 Hz, did not lead to an increase in these frequency bands. It also does not explain why a DC stimulation during stage 2 sleep reportedly effected an increase in visual dream reports although, in this case, the effect was apparently small and, according to the authors, possibly due to arousals and short awakenings (Jakobson et al. 2012b). At this point, we speculate that lower gamma band frequencies lead to a visible effect because they are linked to the unfolding of secondary consciousness in dreams.
The most striking finding was that subjects reported the ability to “see myself from the outside” and to “watch the dream from the outside as if it was displayed on a screen”. These items belong to the factor DISSOCIATION. Apparently, our subjects took on a third-person perspective following lower gamma band stimulation but not stimulation in any other frequency (2 Hz, 6 Hz, 12 Hz, 70 Hz, 100 Hz) or sham (no current applied).
However, although we were able to induce secondary consciousness in dreams through stimulation with 40 Hz, a similar though smaller effect was observed for stimulation with 25 Hz. Surprisingly, 25 Hz stimulation was associated with CONTROL over the dream plot, whereas stimulation with 40 Hz was not. This finding suggests that specific brain rhythms may be directly linked to cognitive functions and that we have just begun to discover their potential.
Surprisingly, we found no evidence of theta-gamma coupling, as would be expected from NREM sleep studies (Marshall et al. 2011). At present, we think this may be related to the fact that NREM sleep is highly synchronized, perhaps facilitating such coupling, whereas NREM sleep is desynchronized. As is often the case in science, answering one question generates several others. We will continue to search for answers and also look forward to the extension of our studies by other laboratories.
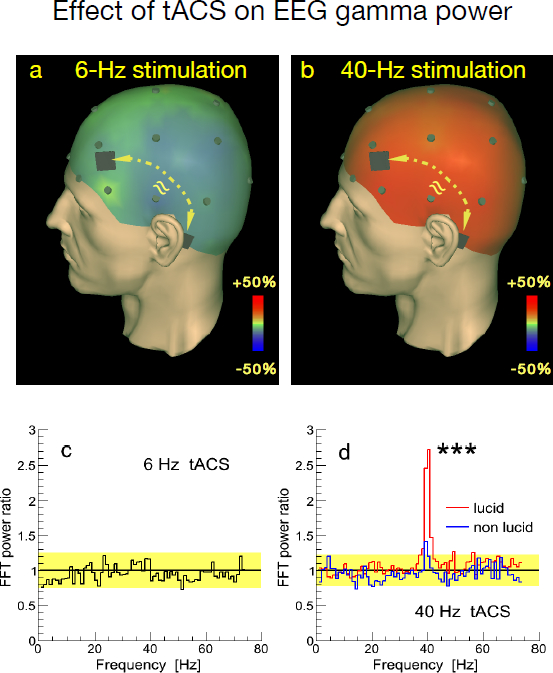 Figure 6: Effect of transcranial alternating current stimulation (tACS) on EEG gamma power. tACS electrodes were placed bilaterally at frontal and temporal positions (black rectangles) and current flowed back and forth between these electrodes. EEG electrode placements are indicated as dark dots.
Figure 6: Effect of transcranial alternating current stimulation (tACS) on EEG gamma power. tACS electrodes were placed bilaterally at frontal and temporal positions (black rectangles) and current flowed back and forth between these electrodes. EEG electrode placements are indicated as dark dots.
a)Stimulation with 6 Hz resulted in no change in lower gamma activity around 40 Hz (37–43 Hz).
b)Stimulation with 40 Hz led to a strong increase in lower gamma activity around 40 Hz.
c)Grand average Fast Fourier Transform (Fast Fourier Transform, FFT) power ratios of activity during vs. activity prior to stimulation for the 6 Hz stimulation condition. Yellow shading represents mean values 2 standard errors (s.e.). Any excursions outside of this range would be considered significant at least at the p < .05 level. However, with 6 Hz, we see no significant stimulation-induced increase in 6 Hz activity.
d)Grand average FFT power ratios of activity during vs. activity prior to stimulation for the 40 Hz stimulation condition. Yellow shading represents mean values 2 standard errors (s.e.). Note that lucid dreams (red line) are accompanied by a significantly larger increase in the 40 Hz frequency band than non-lucid dreams (blue line) (independent two-sided t tests between lucid and non-lucid dreams during stimulation with 40 Hz: t40Hz = 5.01, df = 35, p < 0.001).
3.9 Brain Correlates of Lucidity and a Neuropsychological Model.
Our findings of frontal cortical EEG activation to a level intermediate between non-lucid dreaming and waking is compatible with the hybrid state formulation derived from subjective data. More specifically, we attribute the findings to sufficient activation of executive ego functions in the frontal lobes (Baddeley 1992; Goleman & Davidson 1979), but not so intense an activation as to disenable the REM sleep generator in the pons and posterior thalamocortical brain that is the physical substrate of dreaming. This formulation is resonant with the oft-repeated complaint that dream lucidity is difficult both to attain and maintain. The hybrid state of waking and dreaming is thus both rare and fragile, suggesting that it is not an adaptive condition for survival and has been eliminated, or reduced to a very low level, by evolution.
It is not difficult to imagine why it would be maladaptive to program waking and dream consciousness at the same time. We will come back to this consideration when we discuss clinical implications below, but at this point we wish to stress the winner-takes-all model that we have sketched as the protoconsciousness hypothesis (Hobson 2009). According to that model, both waking and dreaming are states of consciousness engendered by specifiable brain mechanisms. Waking is governed by aminergic dominance, and dreaming by cholinergic dominance, but both states depend on suppression but not total obliteration of the other. Waking and dreaming are competitive and cooperative brain-mind states.
Of course there is more to the neurophysiology of the differential brain mediation of waking and dreaming. In addition to the chemical neuromodulation mentioned above, we know that REM sleep dreaming is mediated by the active inhibition of both sensory and motor input and output. The data from our studies of lucidity now further suggest that the two states are also differentiated by regional activation of the cortex. Waking and lucid dreaming are both favored by strong 40 Hz power in the frontal EEG, indicating that frontal lobe activation is a critical mediator of both waking and lucid dream consciousness. Because this sort of activation has been found to correlate with lucidity, we hypothesize that it mediates the wake state component of lucidity. This supposition is also supported by the finding of frontal lobe inactivation in REM sleep, which is correlated with non-lucid dreaming (Braun et al. 1997; Dang-Vu et al. 2007; Desseilles et al. 2011; Nofzinger et al. 1997).
An additional nicety of the theory is that the voluntary eye movements by which lucid dreamers indicate their awareness of their conscious state to third-party observers (Hearne 1978; LaBerge 1980) is evidence of frontal eye field activation in lucid dreamers. This volitional override of the brain stem saccadic eye movement generator is further evidence of the change in the balance of brain-power in several states of consciousness. In lucid dreaming, the wake state control of gaze is returned via frontal lobe activation. According to Metzinger (2013), this is tantamount to the activation of an “epistemic agent model” (EAM), a representation of the self as knowing. This would seem to clinch the argument that conscious states are electrophysiologically differentiated and explained by neurophysiology. This is not surprising, but its specification has been greatly advanced by the scientific investigation of lucid dreaming. A speculative hypothesis that we believe must be tested is that waking entails not only frontal lobe dominance in mediating thought and top-down eye movement control, but that the brain stem itself is primarily harnessed to the analysis of external data with relative suppression of its internal program (see also Activation-Input Gating-Modulation, AIM model, Hobson 1992).
Unfortunately we have no animal model for dream lucidity because we have every reason to suppose that reflective insight such as observed in lucid dreaming necessitates sufficient language capacities assumed essential in the formation of abstract thought (Einstein 1941) or reporting of such. For this reason, we assume that infra-human mammals, which lack significant language capability, cannot become lucid or report their non-verbal dreams. Whatever one thinks about animal dreams (and we suppose that primary consciousness does accompany their very elaborate REM sleep), no one believes that they are capable of verbally reporting their subjective experience. Dogs and cats do, however, whimper, twitch, and run in their sleep (Lucretius 1995), lending credence to the hypothesis of primary dream-consciousness in animals other than human beings. Animals may dream, and they may become lucid in their dreams, but we doubt the latter and can never offer scientific judgment about either possibility.
The exploration of the physiology of primary consciousness is in its infancy and can be expected to flourish in the future even if we have only rats for subjects (Datta & Hobson 2000; Datta & MacLean 2007). But if we want to learn more about secondary consciousness, we will have to put up with rather severe limitations (Dresler et al. 2012). We trust that advances in brain imaging technology may help this situation. Meanwhile, we hold that the study of lucid dreaming, however difficult, conveys insights about the brain basis of consciousness that is obtainable in no other way.