4 Constitution and constraint
We can most readily see why this case represents an interesting challenge for componential mechanism by fitting it to the four steps outlined in section 1, above.
Identify the phenomenon of interest ψ
Identify the system S that ψs
Identify the relevant spatial sub-parts {X} of M (and their relevant intrinsic properties)
Describe how the parts {X} are organized such that S ψs
The specific phenomenon of interest ψds is direction selectivity or, more precisely, the release of neurotransmitter in and only in response to motion in a specific centrifugal direction. The system Sds that exhibits ψds is the dendrite of the SAC. It is also easy to say what the parts {Xds} of the mechanism Mds are in virtue of which the dendrite ψ-dss, and how they are organized. I have provided that sketch above. Finally, it seems right to say, following Craver (2008), that the relationship between Mds and its parts {Xds} is one of componential constitution, such that all the parts {Xds} are at a lower level than Mds, and together constitute Mds. But now it gets interesting for componential mechanistic explanation as currently developed. For only some of the parts of Mds—including the voltage gated calcium channels, and the input and output synapses—are at a lower (spatial) level than the dendrite Sds. The inhibitory dendrites of the neighboring SACs are at the same level as Sds, the bipolar cells and their spatial relations are arguably at a higher level than Sds (although one might wish to screen these off as mere inputs to the mechanism), and the mechanism M as a whole in virtue of which Sds ψ-dss is certainly at a higher level than, and is in no way a physical or functional component of Sds.
I think this example demonstrates that not every mechanistic explanation will have the “bottom-up” or “level-restricted” character that the mechanism for the action potential has, where function is built entirely from the capacities of lower-level components and their interactions. In the SAC dendrite, we appear to have a case not of a system that ψs in virtue of the capacities and relations of its components (and that could in turn be thought of as a component supporting the activities of a larger functional system), but rather very nearly the reverse: a system that ψs in virtue of the properties of and interactions in the higher-level system of which it is a part. That is, the SAC dendrite is not functionally related to its surrounds as a component to a higher-level system; nor is the higher-level system related to the SAC dendrite as one of its components. Instead, I want to say that the higher-level mechanism M acts as an enabling constraint on S.
Before providing a bit more in the way of substantial analysis of the concept of an enabling constraint, let us pause to consider one way in which a supporter of componential mechanistic explanation might resist this conclusion by redefining the system Sds to include the mechanism Mds. I think this is not a viable option for a number of reasons. First, it would appear to violate standard usage: neuroscientists speak of direction-selective dendrites, and not of a directionally selective network spanning several retinal layers. The debate in the neuroscientific literature concerns not the definition of the direction-selective system, but the relative role of intrinsic and extrinsic mechanisms for dendritic direction selectivity in SACs (Hausselt et al. 2007; Lee & Zhou 2006).
Second, it appears that the mechanism as a whole is not direction selective. Any given SAC, for instance, and certainly the network as a whole, signals motion in all directions. Even if we restrict the definition of Mds to the entities in virtue of which one particular SAC dendrite is directionally selective, the symmetry of the mechanism—the fact that SACs mutually constrain one another and the same bipolar cells synapse onto more than one SAC dendrite—strongly suggests that very same mechanism generates right direction selectivity in the rightward-reaching dendrite in SAC0, and left direction selectivity in the leftward-reaching dendrite in SAC2 (e.g., in Figure 4). The mechanism, that is, does not have the same direction selectivity as either of the dendrites. Rather, it’s as if when you turn the crank one way (i.e., the stimulus moves one way) the mechanism produces one output; and when you turn it the other way, it produces the other output.
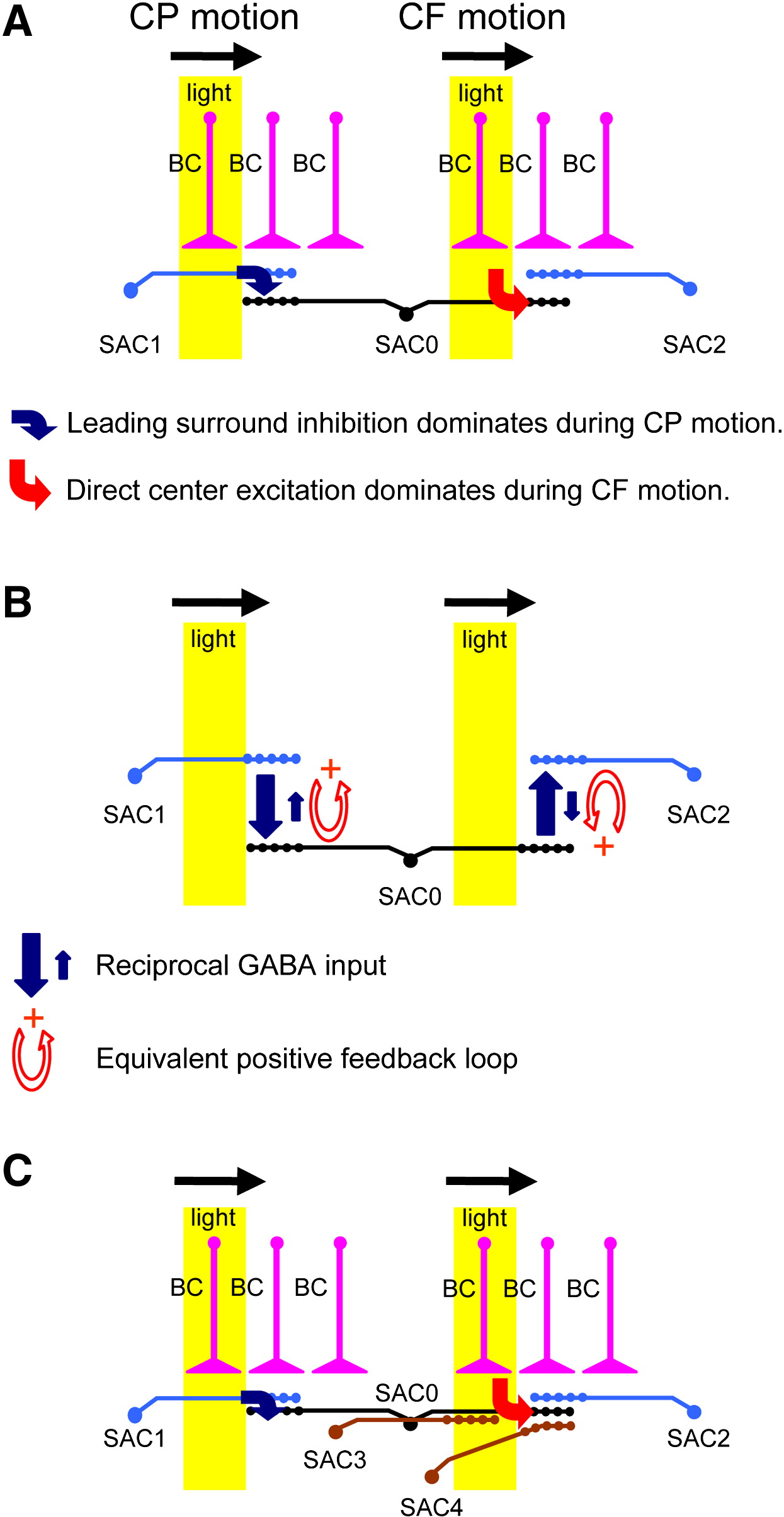 Figure 5: Lateral inhibition between neighbouring SACs contributes to direction selectivity in the dendrites. Reprinted from Lee & Zhou (2006).
Figure 5: Lateral inhibition between neighbouring SACs contributes to direction selectivity in the dendrites. Reprinted from Lee & Zhou (2006).
This suggests a different way to illustrate the limitations of componential mechanism as formulated. Craver writes that the explanandum phenomenon ψ is “typically some behavior of the mechanism as a whole” (Craver 2008, p. 139), and he thus might insist, contra my way of formulating his framework in 1, that it is the mechanism M and not the system S that exhibits ψ. In this case, because I have agreed that the parts {X} in fact constitute M, any conflict between functional and spatial levels disappears. But in the case before us it seems that the mechanism responsible for, say, rightward direction selectivity does not in fact exhibit rightward direction selectivity. So the functional puzzle reasserts itself in a different guise.[6]
One might nevertheless insist on distinguishing these mechanisms in subtle ways—perhaps Mds0 includes these synapses from bipolar cells, but not those synapses, while Mds2 includes those synapses but not these. I doubt whether this can work, because explaining direction selectivity in either direction will require reference to the excitatory inputs from bipolar cells to the centrifugal dendrite, and the inhibitory inputs from the overlapping centripetal dendrite, which are in turn a result of the excitatory inputs from the very same bipolar cells synapsing onto the centrifugal dendrite. But let us take the possibility as granted. Then one seems forced to say something along the following lines: the mechanism as a whole ψs, but signals ψ-ing with the dendrite.
Let us consider this possibility carefully. As I intimated above, scientists debate the relative importance of intrinsic and extrinsic mechanisms for dendritic selectivity in SACs. Hausselt et al. (2007) note that direction selectivity in SAC dendrites persists in the presence of GABA and glycene receptor antagonists, which would deactivate the portions of the normal mechanism that involve mutual inhibition between neighboring SACs. In these circumstances, one might argue that only the portions of the original mechanism intrinsic to the dendrite matter in the explanation of direction-selectivity, and in such a case it is clearly the dendrite that ψs. What shall we say, then, when we remove the antagonists from the system and reapply the same directional stimulus, resulting in neurotransmitter release from this dendrite? One option is: whereas before the dendrite ψ’d, now it merely signals the ψ-ing of the larger mechanism. But it seems clear to me that, if the dendrite can ψ, then adding network interactions that aid and enhance (that is, do not in any sense prevent) ψ-ing can hardly cause it to not ψ, but only signal ψ. This points to a fourth and final reason to reject the general move to extend the neural system S to include the mechanism M whenever it is (or contains entities that are) on a higher level than S: one would apparently need the ability to rigorously distinguish between ψ-ing and signaling ψ in an overall system where to ψ is generally also to signal it—that is, where signaling and doing are deeply intertwined. Thus, I believe we must insist: the dendrite ψs.
For all these reasons, I do not think it is wise to hold onto level-restricted explanations and componential composition by fiat. Instead, it is time to expand the scope of mechanistic explanation by considering the various ways in which systems S relate to the mechanisms M that enable their activities. I think the case of SACs is especially important because it illustrates one way in which local selectivity in parts of a network can be the result of the interplay of excitation and mutual inhibition between non-selective parts of that network, which is clearly something that we need to understand better if we are to accurately characterize the functional mechanisms at work in both small and large-scale brain networks (Anderson et al. 2013). But other structure-function relationships appear to call equally for a broader account of mechanistic explanation. For instance, the direction-selective ganglion cell DSGC (Direction-Selective Ganglion Cell), mentioned briefly above, responds to stimuli moving only in its preferred direction (which of course varies cell-to-cell). In this case, there do not appear to be any intrinsic mechanisms for the direction selectivity of the DSGC. Rather, SAC dendrites selectively synapse onto DSCGs with preferred stimuli antiparallel to the SAC dendrite preference (Briggman 2011) thus suppressing responses to motion in the non-preferred direction. DSCGs seem to simply inherit their selectivity via their synaptic contact with SACs—and, in fact, elimination of SACs from the retina abolishes direction selectivity in DSCGs (Yoshida et al. 2001). Here I just don’t see any case for a compositional relationship between the mechanism (or its parts) and the selective system. Instead, the relevant mechanism synapses onto the relevant system, and by suppressing a sub-set of its response tendencies, induces selectivity.
This brings us finally back to the notion of “constraint”, which I think may help us understand the full range of mechanism/system relationships in the brain. The term constraint has been used in myriad ways in the literature on scientific explanation. In evolutionary biology, scientists refer for instance to stability constraints (Schlosser 2007) and both universal and local developmental constraints on evolvability (Maynard Smith et al. 1985). There are also law-like constraints on the possible states of physical systems generally (Lange 2011). None of these capture the sense of “constraint” that will be most helpful to us here.
One notion that gets us close is the idea of a “capacity constraint”, that is, a limitation on the capacity of a process that might take the form of changing the relative probabilities of the range of possible process outcomes (Sansom 2009). This certainly has the right flavor, for in the mechanism under discussion above it appears that the excitatory and inhibitory interactions between bipolar cells and neighboring SACs bias the outcome of the dendritic processing of the moving stimulus. But insofar as a capacity constraint is generally conceptualized in terms of the reduction of some pre-existing whole ability—in Sansom’s (2009) example, being handcuffed limits one’s ability to move one’s hands—this does not offer quite the right organizing frame for explanation in neuroscience.
The reason is that in the neurosciences we want to understand not just the capacities of entities, but how the structured interactions between entities give rise to functions, which are, crucially, differential and differentiating processes (that is, they differ from one another, and they differentiate between stimuli). Capacities in the sense of general powers (the capacity to generate an action potential, say) are necessary conditions for functions, but they are not yet functions; the DSGC is strictly speaking non-functional in the absence of SACs, even though it will continue to exercise its capacity to fire action potentials in response to inputs from bipolar cells. Constraints of the sort under investigation here serve to limit capacities, but in so doing they enable functions; they result in an enhancement (not a reduction) of the abilities of the system (and the organism).
For this reason I propose to analyze the general functional (and, crucially, non-hierarchical) relationship between mechanisms and systems in the following way: an enabling constraint is a relationship between entities and/or mechanisms at a particular level of description and a functional system at the same or a different level, such that the entities/mechanisms bias (i.e., change the relative probabilities of) the outcomes of processing by the system. Such enabling constraints offer necessary but not sufficient conditions for the instantiation of differential function in neural systems. Because enabling constraints are synchronic rather than diachronic, the idea shares the same explanatory advantage that the relation of constitution has over the relation of “causation” (when understood, e.g., as an event involving the transmission of some property, power, or conserved quantity from one entity to another). As Craver & Bechtel (2007) point out, such a conception of causation does not accomodate interlevel functional relationships well, because these are often synchronic and symmetric, whereas causation of this sort is temporal and asymmetric.[7] In addition, enabling constraints can be mutual, which gives the idea an advantage over both causation and constitution as an analysis of functional relationships in the brain.
Enabling constraint =Df A physical relationship between a functional system S and entities {X} (and/or mechanism M), at the same or different level of description, such that {X} (and/or M) changes the relative probabilities of various possible functional outcomes of activity in S.
To understand function not just in systems like SAC dendrites and DSGCs, but also in the large scale networks that are partially constituted by the Transiently Assembled Local Neural Subsystems TALoNS (Transiently Assembled Local Neural Subsystems) crucial to the functioning of a dynamic brain (Anderson 2015), we need to accept that there is a broader range of relationships that mechanisms can have to functional systems, beyond componential constitution. Function in TALoNS results not from structured interactions between stable, autonomous low-level components, but rather from the interplay between the capacities of lower-level entities and higher-level network dynamics. That interplay, I argue, is best analyzed in terms of the mutual constraint that exists between bottom-up and top-down, feed-forward and feed-back mechanisms in the brain.